The U.S. Food and Drug Administration (FDA) has issued a public warning about a potential norovirus oyster recall involving frozen half-shell oysters imported from Tongyeong, Gyeongsangnam-do, South Korea. The agency is urging consumers and distributors to immediately stop consuming and distributing the product.
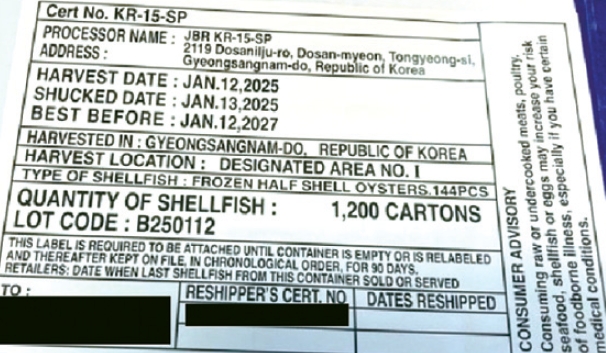
The oysters were harvested on January 12, 2025, and processed by local company JBR (Facility No. KR-15-SP). The product is labeled with Lot No. B250112 and has an expiration date of January 12, 2027.
Imported by WANG Globalnet, a Korean American food distributor based in Vernon, California, the oysters were primarily shipped to California, Arizona, Colorado, Montana, and Utah. However, the FDA noted that other states may also have received shipments.
A case of norovirus infection was reported in Utah on July 16, after a person consumed the product. According to the Utah Department of Agriculture and Food, the individual experienced typical norovirus symptoms such as diarrhea, vomiting, abdominal pain, and fever. In response, WANG Globalnet initiated a voluntary recall of the product on July21.
Norovirus is highly contagious and can cause illness within 12 to 48 hours of exposure. It poses significant health risks, especially for children, the elderly, and individuals with underlying conditions, due to the risk of dehydration and complications.
The FDA advises consumers to check labels and discard any oysters marked with the affected lot number. Those showing symptoms should seek medical care immediately.
BY HANKIL KANG [kang.hankil@koreadaily.com]



